Bringing medicines manufacturing into the future: an update on the Medicines Manufacturing Innovation Centre
Two years in, the Centre continues to make waves. The facility is a unique partnership that will revolutionise medicines manufacturing.

Business Development Director

Founded upon a collaboration between CPI, University of Strathclyde, UK Research and Innovation, Scottish Enterprise and the founding industry partners, AstraZeneca and GSK, the Medicines Manufacturing Innovation Centre allows industry, academia, healthcare providers and regulators to work together pre-competitively to solve challenges and de-risk new technologies, providing a clear pathway for their widespread adoption within the pharmaceutical industry.
The Centre will enable and accelerate the development of next-generation medicines manufacturing technology while maximising opportunities within the medicines supply chain.
Addressing industry challenges
While 2020 was certainly a year like no other, the Medicines Manufacturing Innovation Centre has continued to innovate. The year as been defined by the addition of four new partners to the consortium, the official ground-breaking of its state-of-the-art facility, and the continued development and success of its flagship ‘Grand Challenges’. These ‘Grand Challenges’ are the initial focus of the Centre and are effectively large strategic projects which overcome industrial hurdles limiting pharmaceutical development. The initial challenges cover the efficient production and delivery of oral solid dose medicines.
When compared with traditional ‘batch’ manufacturing processes, continuous manufacturing allows for greater control and a more efficient use of time. Grand Challenge 1 will define how oral solid dosage medicines can be produced more robustly and efficiently utilising continuous direct compression (CDC). The aim of Grand Challenge 1 is to ultimately enable pharmaceutical companies to develop formulations quicker and at a lower cost.
The manufacturing of medicines for clinical trials represents a significant – but essential – investment for drug manufacturers. The way this supply is produced currently relies on a ‘just in case’ approach. This means that requirements are predicted up to two years in advance to allow for lead times, and drugs are often over-produced to ensure sufficient supply. Grand Challenge 2 focuses on the delivery of ‘Just in Time’ clinical supply material to patients through the development of an innovative, automated supply chain platform; thus, enabling effective late-stage customisation of trial stocks. The Pharmacy Automation for Clinical Efficiency (PACE) platform will allow for the production of multiple drugs on a single line, individualised packaging and real-time quality checks, reducing waste and risk all while increasing speed to patient.
Delivering next-generation manufacturing
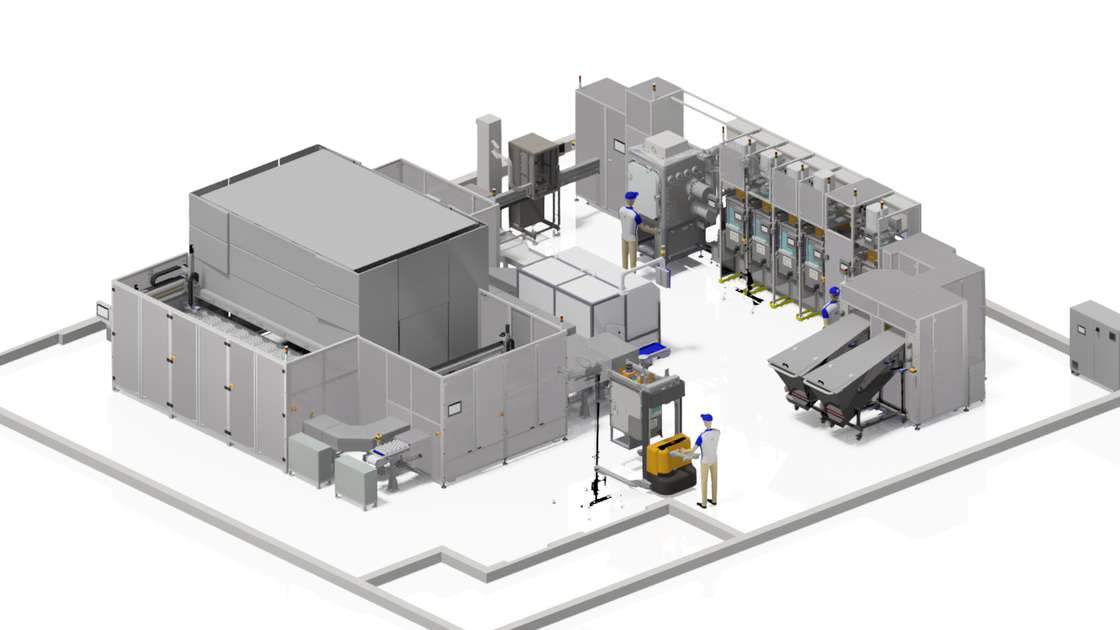
As well as delivering capability, the Medicines Manufacturing Innovation Centre is also delivering a physical facility. At the end of October 2020, the team broke ground at the official site of the Medicines Manufacturing Innovation Centre in Renfrewshire, Scotland. This bespoke facility has been designed to house the Centre’s ongoing and planned Grand Challenge projects, along with innovative design elements that can accommodate future programmes. In addition, ‘future proofed’ design elements, such as adaptable spaces, set the Centre apart from more routine pharmaceutical facilities. This facility will enable partners to work collaboratively and flexibly, addressing challenges from across the medicines supply chain to introduce transformative solutions into pharmaceutical manufacturing. Expected to house over 80 staff in both technical and non-technical roles, the facility is due for completion later this year and will be operational in early 2022.
A collaborative approach
The four new partners who have been introduced over the last few months will both strengthen and diversify the range of expertise in the consortium. Joining the Centre in August, Perceptive Engineering LTD and Process Systems Enterprise LTD (PSE) will contribute to the development of a digital manufacturing solution to support Grand Challenge 1. In September, it was announced that Applied Materials, Inc. was joining this partnership, supporting Grand Challenge 2 through the integration of its SmartFactory Rx® automation software into the Centre’s clinical supply platform. We are also very lucky to have the support of Siemens and their technology across both of our grand challenges. Our unique partnership model helps introduce innovative technology to medicines manufacturing through our cross-sector partners. We are continually welcoming new partners to strengthen our knowledge-base, introduce innovative technologies and fulfil both current and future Grand Challenges. Several new partners from leading global organisations have recently committed to joining the collaboration; with their contributions to be announced in early 2021.
We are actively looking for potential collaborators to work with us on Grand Challenge 2 to accelerate the implementation and robustness of this technology, which will improve the distribution of clinical trial drugs to patients. The inherent uncertainty within the clinical trial process means long lead times and that stock quantities must be estimated up to 24 months in advance. At the moment, release authorisation of this stock is carried out by a Qualified Person (QP) who must collect and review the relevant data. The Centre aims to improve industry and patient outcomes by increasing efficiencies with the use of digital quality approaches and automated technology.
Future growth
The technologies developed through Grand Challenges 1 and 2 will enable materials efficiencies and speed-up timelines to achieve just-in-time, right-first-time and real-time-release manufacturing principles.
In addition, we are beginning to seek new partners for our upcoming Grand Challenge projects, in particular Grand Challenge 3, which will focus on state change technology for oligonucleotide manufacture. If you are interested in being a part of the Medicines Manufacturing Innovation Centre or are looking for more information, please contact me on neil.sheddan@uk-cpi.com

The Medicines Manufacturing Innovation Centre
The Medicines Manufacturing Innovation Centre (MMIC) enables the acceleration of next-generation medicines manufacturing technology. By allowing cross-sector and cross-discipline collaborators to get together in a non-competitive environment, innovations that have the potential to revolutionise the pharmaceutical industry can be introduced with reduced risk. Ultimately, this will allow for more efficient drug production, protecting future generations by bringing new medicines to market safely and quickly. With many years to come in this collaboration, the potential is endless.
Learn more about the Medicines Manufacturing Innovation Centre.
Enjoyed this article? Keep reading more expert insights...
CPI ensures that great inventions gets the best opportunity to become a successfully marketed product or process. We provide industry-relevant expertise and assets, supporting proof of concept and scale up services for the development of your innovative products and processes.