How digital technologies could help solve the pharmaceutical industry’s supply chain challenges
How digital technologies could help solve the pharmaceutical industry’s supply chain challenges

Director of Digital Business Systems

Is it possible to manufacture medicines for clinical trials more effectively while reducing waste? The answer could lie in an innovative, digitally-enabled robotics platform.
As the global population’s life expectancy continues to increase, demand for treatments targeting long-term and complex conditions (such as diabetes, nervous system disorders and heart disease) is rising. The healthcare sector is fighting to reduce the significant but essential investment associated with development of medicines for such treatments and to reduce inefficiencies in the supply chain.
Addressing these challenges calls for a unique, cross-sector approach, such as the one offered by the ground-breaking Medicines Manufacturing Innovation Centre. I am proud to be a part of this pioneering collaboration, founded by CPI along with partners from Big Pharma – AstraZeneca and GSK – Government funders (Scottish Enterprise and UK Research and Innovation) and academic stakeholders (University of Strathclyde). This partnership aims to bring together multidisciplinary expertise to create innovative pharmaceutical manufacturing solutions in a pre-competitive and mutually beneficial environment.
The Medicines Manufacturing Innovation Centre (MMIC) has developed a number of Grand Challenges, which aim to collaboratively tackle industrial hurdles in medicines manufacturing. My role is to lead the strategy, delivery and realisation of Grand Challenge 2, which focuses on delivering oral medications to patients with minimal waste and maximum speed. My colleagues and I are utilising digital technologies embedded in the Pharmacy Automation for Clinical Efficiency (PACE) platform to facilitate Just in Time (JIT) trial stock manufacture – producing medicines for clinical trials when and where they are needed to a specific order.
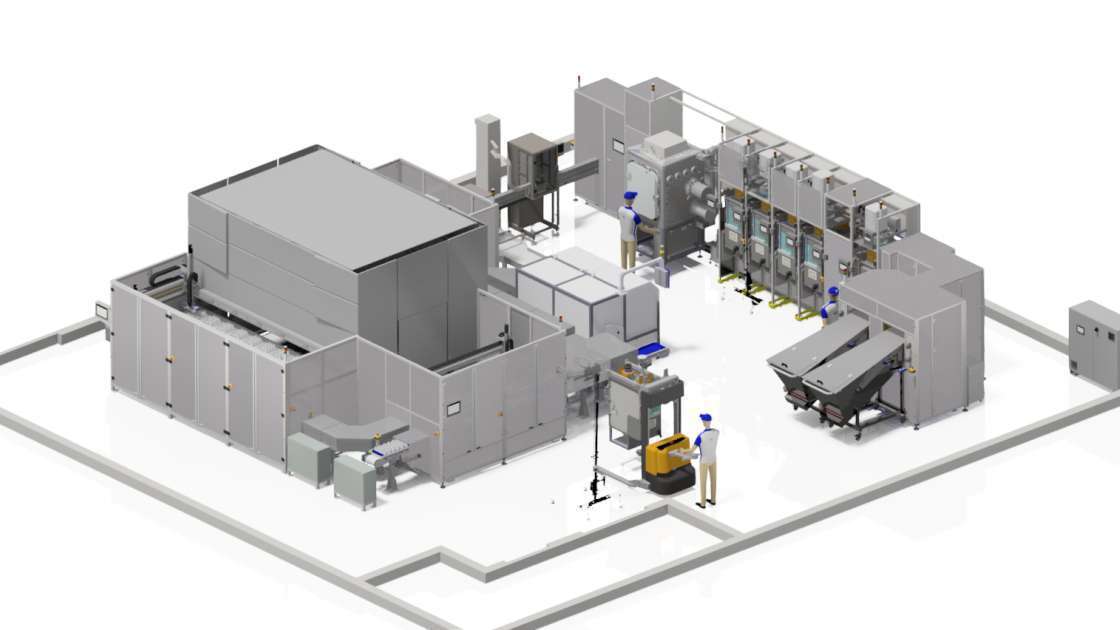
The PACE platform
The PACE platform is a digitally-enabled (software and hardware) automated line, designed to meet orders for multiple products, packaging and labelling drugs in the same facility without cross-contamination. Fundamentally a conveyor belt with robots rapidly filling, sealing, cataloguing and dispensing bottles of medicine with the software infrastructure to then release the products.
At present, manufacturers and clinical trial planners are forced to predict volumes of medicines up to two years in advance to ensure that they can meet the needs of the clinical trial strategy. Unfortunately, these predictions cannot accurately account for fluctuations in demand or changes in the clinical trials themselves. As a result, larger volumes of clinical trial drugs are produced ‘just in case’. Due to changes in demand and product expiration, many of the products are left unused.. The digitally-enabled features of our PACE platform aim to deliver greater availability, faster production and adaptive supply strategies of clinical trial medicines by realising JIT manufacture.
One exciting feature, currentlyin development, is the platform’s real-time Qualified Person (QP) dashboard. This software has been designed to help ease the authorisation and decision-making process when distributing drugs to clinical trials; although the long-term desire is to apply this beyond clinical trials. Currently, QPs are based in facilities and release batches through thorough review of records of relevant data; from multiple spreadsheets or paper records to complete the legal certification process. Our platform’s dashboard could save considerable amounts of time by automatically compiling relevant data into a digitally accessible system, while medicines are packaged and labelled. This allows accurate data to be collected (and maintained in real time) and for QPs to access the relevant data quickly and remotely. The dashboard could additionally flag certification issues immediately and cease the manufacture of products in question, instead of overproducing, resulting in unused stock.
An additional aspect that the PACE platform will unlock is the potential to connect the manufacturing data train (from PACE) to Internet of Things (IoT) devices. “Smart” labels (as an example of IoT technology) could further cut excess waste and cost in pharmaceutical supply chains. These innovative labels will utilise technology; most smartphone users will recognise: Quick Response (QR) codes and Near-Field-Communication (NFC). These smart labels could help prevent any wastage caused by loss during transport and storage. They can help manufacturers and healthcare professionals monitor the location and the environmental conditions (i.e. temperature and humidity) that stock is exposed to, which has the potential to affect drug efficacy.
Conclusion
The digitally-enabled features of the PACE platform, such as the real-time QP dashboard and smart packaging system, will allow for more cost-efficient manufacture of drugs with increased responsiveness to fluctuations in demand and transport conditions. Although such digital solutions are certainly not a quick fix for today’s healthcare challenges, they could significantly accelerate the current clinical trial process – ultimately helping healthcare adapt to the needs of our global population.
Read more about this project and the platform’s innovative features here: https://www.europeanpharmaceuticalreview.com/article/122191/the-circuit-of-life-saving-on-manufacturing-waste-and-delivery-related-supply-chain-inefficiencies/
Enjoyed this article? Keep reading more expert insights...
CPI ensures that great inventions gets the best opportunity to become a successfully marketed product or process. We provide industry-relevant expertise and assets, supporting proof of concept and scale up services for the development of your innovative products and processes.