In-vitro diagnostic medical devices
Transforming your idea into a clinically relevant tool


In-vitro diagnostic devices
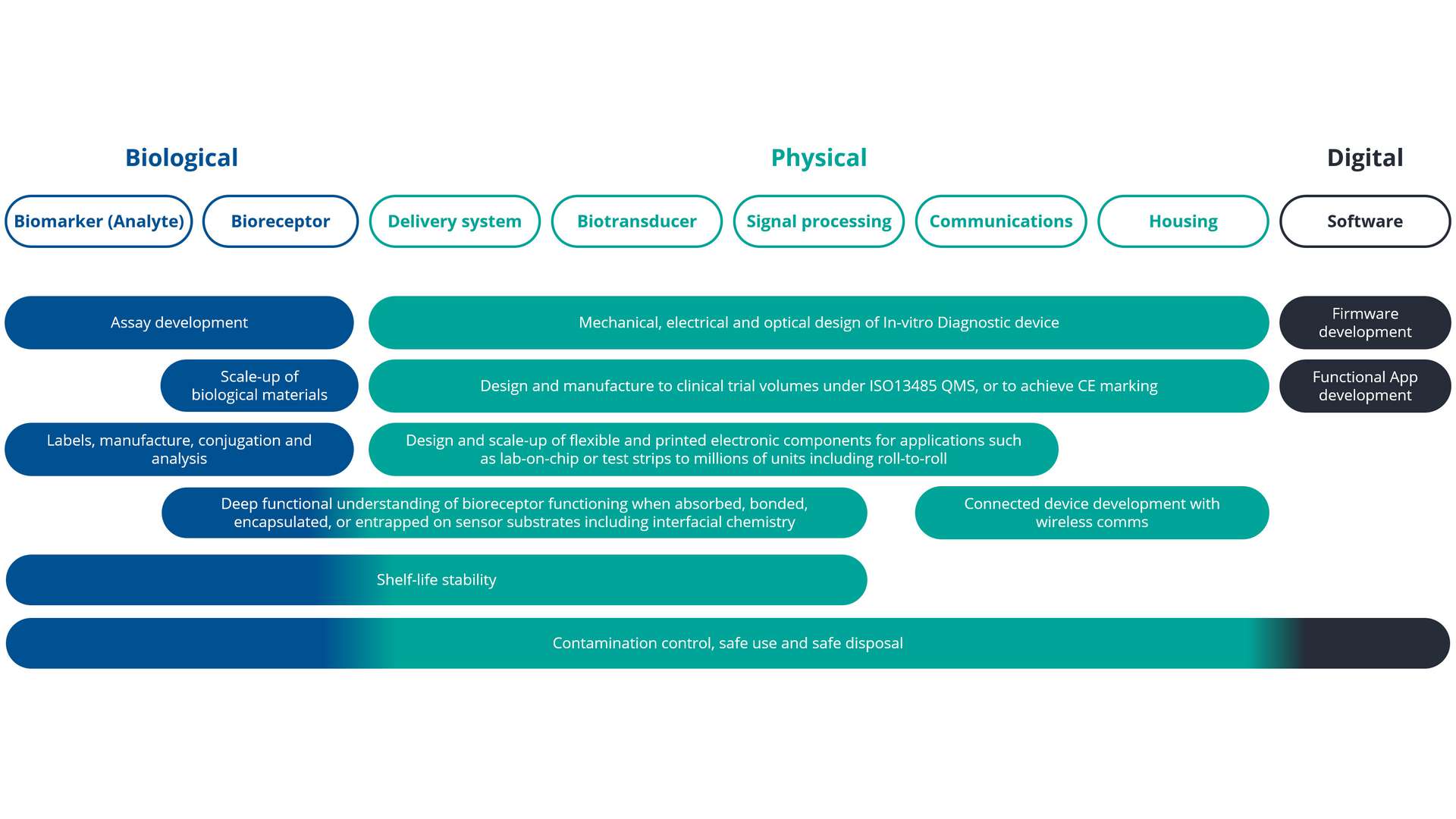
Developing, testing and deploying commercially successful In-vitro diagnostics (IVD) is a multidisciplinary challenge. In order for a device to be successful we need to bring together a number of complex components including biological materials, physical delivery methods, sensing, signal amplification and often digital elements to enable integration with apps and digital healthcare records. Further complications are added by the fact that these elements must be brought together to function as one, under the correct regulatory conditions. And it doesn’t stop there, device developers need to be connected into the right individuals and groups within the healthcare ecosystem such as patient groups and healthcare providers to help with market adoption.

At CPI we have multidisciplinary teams with a broad range of capabilities who can work across the spectrum to drive progress and accelerate the development and scale up of your diagnostic device. We are well connected into the healthcare and innovation ecosystem and can help you to take your concept right through to commercialisation.
Supporting you with horizontal innovation
At CPI, we have expertise in working across the biological, physical and digital space for IVD, as shown in our (non-exhaustive) set of capabilities. Our skilled scientists, engineers and data experts are adept at horizonal innovation, bridging capabilities across the often isolated biological or physical sciences.
Do any of these apply to you?
Support with device development and manufacture.
Would you like to scale up your disposable components or consumables?
We have significant capabilities in roll-to-roll manufacturing for disposable elements of IVD such as those which involve electrodes, sensors or delivery systems. Our world-leading roll-to-roll equipment can produce up to millions of components or sub-systems to provide market seeding volumes. Roll-to-roll manufacturing offers a route to high volume manufacture and can provide benefits of scale. Our experts have worked in the controlled deposition and patterning of materials across many markets including sensing, displays and medtech. We can work with you from the concept stage through sheet to sheet processes and scale up to large volume with sheet to sheet or roll to roll – transferring the process onto you for commercialisation later.

Do you have an assay that you’d like to develop into a device?
We can work with you to develop integrated mechanical, electronic and optical system design and manufacturing under ISO13485 certified quality management systems. We ensure safety and quality are maintained throughout the process, and you can also use our Quality Management System (QMS) to support your technical file development. We can partner with you from concept through to manufacture of your IVD devices, including performance testing for clinical use and CE marking, and manufacturing within a Class 6 clean environment.

Are you developing a connected device (mIoT)?
With a growth in digital connectivity infrastructure, many companies are developing connected medical devices. The internet of things or the internet of medical things (mIoT) is expected to change the way that healthcare systems operate and will give additional support to patients and their carers. We’re experts in medical device development as well as the Internet of Things and can support you in your development of connected IVD from design through to manufacturing.

Would you like to develop IVD delivery systems?
With our world-leading capabilities in high-volume printed electronics, we can help you to develop and scale processes for material delivery. This can include the development of technologies such as droplet-based manipulation through techniques such as electrowetting. We have expertise in printing and patterning of materials onto flexible or rigid substrates and can help you to develop the correct layer quality, microstructure and interfaces.

Do you need support in measuring your analyte-bioreceptor reaction?
We can work with you to develop a solution to measure or monitor the reaction through sensing technologies such as those that measure electrochemical or optical changes. With our expertise in printing and patterning, including fine line lithography, we can develop and scale electrode structures and combine them with the correct sensing systems and signal processing improvements.


Are you looking for support with assay development
Our expertise in assay development means we can de-risk your technology development.
Do you need access to human tissues or materials?
We have a broad range of capabilities to support you to develop your assay and optimise parameters such as sensitivity, limits of detection, dynamic range and specificity. We can enable pre-clinical validation on a variety of human tissues (blood, urine, saliva, sputum, etc.) within our HTA Licensed CL2 Biolab facility.

Are you developing an assay that requires labelling for signal amplification?
If your diagnostic system requires labels such as nanoparticles, enzymes or fluorescent molecules we can help you to not only develop and scale these materials but also conjugate them to your targets. Using our suite of optical tools we can measure the performance characteristics of the labels (optical properties, fluorescence lifetimes). With our confocal microscope suite, we can help you to understand specificity, cellular localisation and other relevant data to support the faster development your IVD device.
Do you need to scale up your analytes?
If your bio-reagent manufacturing scale is not suitable for your addressable market, we can help. We have a full suite of biotechnology and fermentation facilities to develop and scale processes from a millilitre up to ten thousand litres. We can help you with upstream (molecule manufacturing) and downstream (purification) processes to ensure that your molecule manufacturing process is at the correct quality, yield and scale for market entry.

Our capabilities
Talk to us
Have you created an assay that you want to develop into a medical device? Or have developed part of the components or systems, such as a new type of sensor, and you need help to understand which assays it can be used with – we work with companies of all sizes, simply get in touch to speak to one of our experts.
If you’re a large medtech company and you’re interested in new product development that uses novel technologies such as printed electronics or photonics, we’d be happy to have a chat to see how we can help you develop and scale these technologies to help you build your internal business case.
Let’s innovate together
Contact UsLet’s innovate together
To find out more about how we can work together, please enter your details below.


