
Slurry Hydrogenation in a Continuous Flow Reactor
03 Apr 2012
Multiphase hydrogenation using heterogeneous catalyst was investigated in a Corning® Advanced-Flow™ Reactor. Continuous flow reactor performances were evaluated by studying the effects of different operating conditions.
Multiphase hydrogenation using heterogeneous catalyst was conducted in a glass continuous flow reactor from Corning at the Centre for Process Innovation. The effect of temperature, hydrogen molar ratio, residence time, catalyst content and concentration were studied using noble-metal slurry catalyst. Efficient heat and mass transfer allow achieving full conversion with residence time lower than 1 minute and reduced catalyst load, while keeping selectivity in the same range as batch processes. These results show the high potential of Corning® Advanced-Flow™ reactors for multiphase hydrogenations for high volume manufacturing.
In the pharmaceutical and fine chemical industries, most of the multiphase hydrogenation reactions are conducted in large batch or semi-batch reactors, where the catalyst is suspended in the liquid, which is continuously stirred. Hydrogenation falls under the class of reactions which are fast on noble-metals and are usually limited by external mass transfer in conventional reactors (1, 2). These reactions are also highly exothermic in nature which requires effective heat removal (3). Also, the non-uniform temperature distribution in the conventional reactors may develop hot spots on the catalyst surface or may lead to the formation of several byproduct(s) or intermediate(s) which can also react with one another, and further lead to violent decomposition of starting material or partially hydrogenated intermediates (4, 5).
Therefore, good temperature control within the reactor is essential to increase the selectivity of the desired compounds, and also to prevent thermal explosion conditions. Regarding kinetics, for fast hydrogenation reactions the mass transfer effects become predominant over intrinsic kinetics. As a result the true intrinsic kinetic rates of fast hydrogenations reactions are masked by mass transfer limitations in batch reactors leading to reaction times longer than needed for the reaction to be controlled by intrinsic kinetics (6). In recent years, there has been a growing interest in testing micro reactors at laboratory-scale for catalytic hydrogenations. Micro channel reactors with their small transverse dimensions possess extremely high surface to volume ratios and consequently exhibit enhanced heat and mass transfer rates (6). The catalyst has been usually inserted as packed-bed into the micro channels (7 – 9) or applied as a wash coat on channel walls (10). These micro reactors are producing typically from 0.1 to 10 grams per hour and are then very well suited for screening or to produce from milligrams to few grams.
The question is how to benefit from enhanced mass and heat transfer for industrial production up to several hundred tons per year which requires production rates at least 100 times higher. The pressure drop induced by a packed-bed usually prevents increasing the flow rates: Pressures of 8 and 80 bars have been reported for a liquid flow rate of only 1 ml/min (9 – 11). The wash coating techniques are catalyst specific and would require important technical development and resolution of questions on regeneration and recovery. The required investment may not be relevant today for pharmaceutical and fine chemical production.
One solution described in this paper is to implement slurry hydrogenation in continuous flow reactors that are millimetric in hydraulic diameter size thus providing enhanced mass and heat transfer but using reactors that are designed for industrial full-scale production. The use of slurries has the advantage to be very common in the industrial production environment: The ways to handle it are well known and no major change on that topic is introduced by the switch to continuous processing. Corning’s Advanced-FlowTM glass reactor is an assembly of glass fluidic modules connected together through appropriate piping and connectors (11, 12). These fluidic modules are in the form of a plate with fluidic channels that have a hydraulic diameter of around 1 millimetre. The plate is sandwiched between two heat exchange layers (13), thus providing efficient mass and heat transfer performance. The modularity of these reactors allows performing complex chemical processes in Liquid-Gas-Solid phase. These continuous flow reactors can be used from development to production (up to 1000’s T/y) with no change in mass transfer due to a limited numbering up (14) instead of scale-up, which is a critical point in applications for multiphase reactions. A catalytic hydrogenation was studied with this type of continuous flow reactor. It is a three phase reaction with a substrate composed of starting material in an alcohol based solvent, hydrogen gas and a catalyst with particle size in the range of 30 µm. The reactor was operated by CPI, the Centre for Process Innovation, in their flow reaction facility. The conversion, the selectivity, the impurity profile and the catalyst content were studied.
Results and Discussion
The reaction: a selective hydrogenation
This hydrogenation is performed with a noble-metal catalyst and hydrogen gas. The starting molecule is obtained as a solution in an alcohol based solvent. The main potential sources for impurities are side hydrogenations of other functional groups presents in the molecule (e.g. keto group which tends to be reduced in alcohol when the reaction is not controlled). The catalyst used is slurry with an average particle size distribution around 30µm. And the reaction is highly exothermic (H=-400 to ‑500 kJ/mol). This reaction currently performed in batch process was implemented in the continuous flow reactor. Thus, many parameters identified as potential levers to control conversion and/or selectivity were tested. Conversion is defined as the fraction of starting material (stated as a percent) that is converted to all products, including unwanted side-products. Selectivity is defined as the ratio of the desired product formed to the total of all products that were formed.
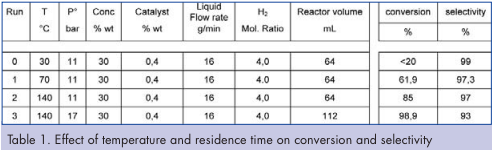
Effect of temperature and residence time Preliminary tests were performed to check hydrogenation feasibility. According to the first results (Table 1), temperature appears to be a lever to control conversion with nearly no conversion at 30°C (batch temperature) and up to 85 percent conversion at 140°C. According to these first experiments, the working temperature range was then shifted from 30°C in standard batch to 100 – 140°C within the flow reactor, above solvent boiling point. Higher conversion rate was achieved by increasing the residence time (run 3). This was performed by the addition of six fluidic modules downstream of the initial reactor. This upgraded reactor is the one displayed in the experimental section.
Effect of hydrogen molar ratio

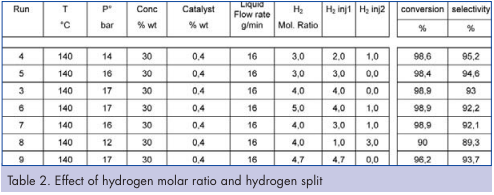
The effect of hydrogen molar ratio on the chemistry with a glass continuous flow reactor was studied by comparing the conversion and selectivity under different molar ratios. Results are presented in Table 2. Various operating conditions could be tested by the control of the quantity of Hydrogen introduced and the flexibility of the split. Results show that conversion levels over 96 percent are obtained, with most cases over 98 percent. And the corresponding selectivity of the product varies between 95 and 89 percent. Higher conversion rates are obtained with higher hydrogen molar ratio except for the last two points. Most of the time, lower selectivity is obtained when separate hydrogen injections are used. It is important to note that the hydrogen split between the separate injections has an impact on the local excess of hydrogen and also significantly influences residence time. And this point, it explains the lower conversion obtained during run 8.
During this continuous production, we were able to observe a bubble régime inside the reactor (Figure 1) which would be expected to give an improved mass transfer. Hydrogen was then separated downstream of the reactor, and can be easily recovered, facilitating industrial application.
Effect of temperature
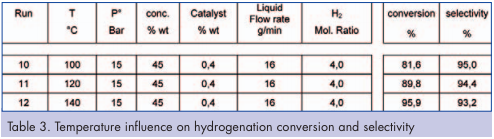
To study the effect of temperature on conversion and selectivity, experiments were carried out in the temperature range of 100 to 140°C. Low conversion is achieved at temperature below 100°C. Results presented in Table 3 indicate that temperature increases conversion, without significant selectivity loss due to short residence time (< 1min). To avoid issues with the use of high temperature with volatile solvent and gas phase, a back pressure regulator was installed downstream the reactor to set minimum pressure at 8 bars. This allowed working at higher temperatures than the solvent boiling point. Depending on operating conditions, the inlet reactor pressure varied from 11 to 16 bars. As a result, we were able to manage this highly exothermic reaction at these pressures and temperatures.
Effect of catalyst content
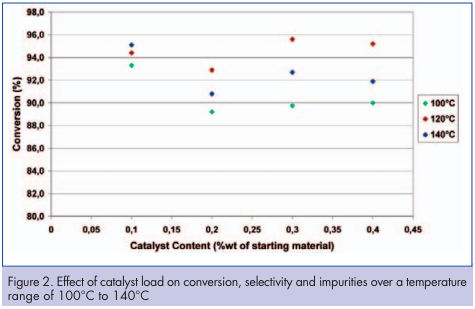
The objective of this set of experiments was to observe the effect of reducing the catalyst charge from the standard 0.4 percent w/w down to 0.1 percent w/w. A base line at 0.4 percent was established before reducing the catalyst charge (Table 3). The trials were conducted at temperatures of 100, 120 and 140°C and are presented in Figure 2. These results show that catalyst content can be significantly decreased. This point is important as it can potentially generate significant costsavings compared to a batch processes. Effect of starting material concentration Comparison between run 3 (Table 2) and run 12 (Table 3) operating conditions shows that different concentrations of starting material can be tested while keeping all other parameters constant. These results showed that high conversion with good selectivity can be achieved at 30 percent and 45 percent by weight starting material. Based on these results, running the reactor at 45 percent would have a significant impact on process intensification and could also avoid the potential concentration step required after the standard batch hydrogenation prior to the following step.
Impurity profile
As mentioned in earlier, a high temperature and a short residence time was used in the continuous flow reactor compared to the standard batch process. Further investigations showed that the amount of impurity was similar to batch and the impurity profiles were almost identical. It has been confirmed through different analytical techniques that there were no heavy compounds.
Experimental
The batch process
During the standard batch process, starting material in alcohol based solution (concentration: 30 percent wt) is introduced in the batch reactor. Catalyst is then added drop wise. Nitrogen purges are performed followed by hydrogen purges at 2 bars. Stirring is then started and the hydrogen pressure is set and maintained at 5 bars. The temperature is maintained at 30°C. After several hours, the reactor is cooled down to 20°C. Once the nitrogen purge is done, the reactive solution is drained and then filtered in order to remove suspended catalyst.
The continuous flow reactor equipment and auxiliaries
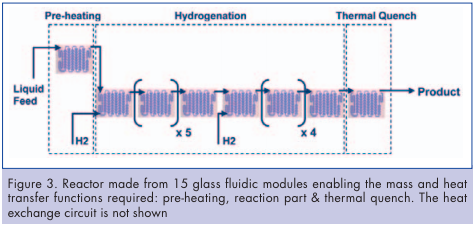
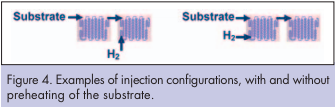
The reactor equipment was an Advanced-FlowTM reactor engineered and manufactured by Corning Incorporated. It consists of an assembly of 15 glass fluidic modules with Heart design cells specifically developed for multiphase applications. This reactor was designed to operate at a total flow rate of a few kilograms per hour, enabling the process development to take place under simple laboratory conditions (Figures 3, 5). During the experiments, temperature is measured on the heat exchange fluid at the fluidic module heat exchange outlet. A thermal quench was installed downstream of the reactor. It consists of a fluidic module whose temperature is set at 20°C. This step is important as it allows an accurate and reproducible residence time management, stopping the reaction instantaneously at the end of the reactor. The reactor internal volume is below 120ml which is in sharp contrast with normal batch equipment which suffers from significant accumulation of chemicals. This allows safer and more stable operating conditions. The flexibility of the equipment allows the testing of different injection configurations. Figure 4 illustrates two examples: In the first case, the substrate is pre-heated prior to hydrogen injection. In the second case, substrate and hydrogen gas are injected together. As a result, the starting material and the catalyst are always in a reducing environment.
It is important to focus not only on the core reaction step but also on feed preparation and downstream process. Therefore, the distribution system used for this hydrogenation employed pre-mixing of the starting material in solvent with the catalyst slurry. This mixture, also called “substrate”, was stirred in a vessel under Nitrogen blanketing and recirculated through a centrifuge recirculation pump. On the recirculation loop a mass flow controlled membrane pump was used to inject the substrate into the reactor (Figure 6). The hydrogen gas flowed through a mass flow controller prior injection within the reactor.
Before starting any experiment, the reactor was run with solvent only, until the temperature reached the desired value. At the same time, by means of a back-pressure regulator downstream of the thermal quench, the pressure was set at 8 bars in order to avoid any vaporization of the solvent at the operating temperature. The feed was then switched to the substrate, and after few minutes, the hydrogen gas was introduced at the desired flow rate. Unlike standard batch processes, no specific purge cycles are required prior starting reaction with continuous flow reactor. The various operating conditions tested were described in the previous section where it can be noticed that different inlet configurations could be tested with one or two hydrogen inlets in order to study the influence of different hydrogen molar ratios and hydrogen residence times. After 10 to 15 minutes stabilization time, the product was collected from the sampling port at regular time intervals. Operating conditions were then changed, and after 10 to 15 additional minutes, another sample was taken.
By this method, a large set of experiments was generated in a short period. The resulting samples were analyzed using High Performance Liquid Chromatography (HPLC) equipment from Agilent with a UV detector. A method was established for the HPLC analysis using peak reagent C4 (Waters) and acetonitrile as the mobile phase, in conjunction with asymmetry C8 column (Waters). Product, starting material and impurities were identified by standards. As all of the products, starting material and impurities have response factors within the same range, all the results were formulated as an area percentage.
Conclusion
Multiphase hydrogenation using heterogeneous catalyst was investigated in a Corning® Advanced-Flow™ Reactor. Continuous flow reactor performances were evaluated by studying the effects of different operating conditions. The reactor was operated for more than 300 hours. The results show that over 98 percent conversion can be achieved with the continuous flow reactor for starting material concentrations from 30 wt percent to 45 wt percent. The catalyst content has “qualitatively limited” to “no impact” in a wide range of concentrations. These last two points open the door to potential savings versus batch reactor processing. The most significant parameters that increase the conversion appear to be hydrogen molar ratio and temperature. Due to flexibility in hydrogen injection, with efficient heat and mass transfer, conversion levels similar to batch processes were achieved with very short residence time without generating new impurities and keeping selectivity in the same range as batch. At the liquid flow rate used during this evaluation, the reactor produces 0.43 Kg per hour. Using higher throughput continuous flow reactors (15) and optimizing the process, it is likely that less than 20 reactors in parallel would be needed to produce 200 tons/year of final product (conversion 98 percent, selectivity 95 percent). These results show the high potential of Corning® Advanced-Flow™ reactors for slurry-based hydrogenation at an industrially important scale.
Let’s innovate together
To find out more about how we can work together, please enter your details below.