
CPI Supports Injectable Protein Purity Programme
26 Jul 2017
CPI has collaborated on a project to improve the purity of injectable biopharmaceutical proteins.
CPI, Arecor and Fujifilm Diosynth Biotechnologies UK (FDBK) have collaborating on a two-year project titled ‘Improved Downstream Operation through Formulation Innovation’, which has been supported by grant funding from Innovate UK’s Industrial Biotechnology Catalyst (IBC) scheme.
The project’s aim was to achieve a step-change in biopharmaceutical yield and quality by improving product stability.
To achieve this, the partners focused on developing novel formulation platforms capable of being applied to routine biopharmaceutical manufacturing to deliver significant improvements in performance.
The specific advancements being sought by industry are improvements in yield, overall protein stability and quality.
The key project focus is on monoclonal antibodies (mAbs), which are the largest and fastest growing sector of the biopharmaceutical industry although some successful testing has also been performed with therapeutic peptides.
The annual production demand for mAb therapeutics is high due to the large number of mAbs approved as therapies through their unique specificity to cellular targets.
Existing major therapeutic applications of mAbs include cancer, chronic inflammatory disease, transplantation, and infection.
The objective
In order to develop a novel formulation platform, the following objective was identified:
To identify smart formulations that confer improved product stability during three areas of downstream processing where protein is most sensitive to degradation: ultrafiltration/diafiltration, product process hold steps, and chromatography.
The activities/outputs
The project used Arecor’s innovative, proprietary formulation technology platform, Arestat™, which has been used to deliver superior biopharmaceutical formulated products.
The platform includes formulation approaches to significantly improve the aqueous stability of therapeutic proteins, peptides, and novel format antibodies.
The industrial bioprocessing experience and expertise of FDBK and CPI, and the high throughput process scale down approaches at CPI, were brought together with the formulation expertise of Arecor to test the application of novel Arecor protein stabilisation formulations to downstream processing.
The following steps of the project have been completed so far:
1. Initial production of purified mAbs by FDBK
FDBK supplied model cells lines to the project using its Apollo™ Mammalian Expression Platform (CHO, DG44-based), which was developed for robust, high levels of recombinant protein expression with manufacturability.
Two model mAbs were produced and purified from FDBK cell lines at FDBK from laboratory bioreactors.
Small amounts of the two purified mAbs were then supplied to Arecor for initial formulation development to stabilise the mAbs against the chemical and physical stresses encountered during downstream processing operations.
2. Identification of formulation shortlist by Arecor
A formulation buffer screen involving more than 400 different formulations of both mAb products were designed and tested under thermal and agitation stresses, which were representative of conditions encountered during bioprocessing.
To be able to prepare such large formulation screens, programmable liquid handling robotics was used to blend and prepare the multicomponent buffers rapidly and accurately.
The use of automation was important for the project given the large number of formulations required to thoroughly screen the design space.
The stability data collected from the high-throughput screens were modelled computationally and used by Arecor to design a shortlist of formulations for application at each stage of the downstream processing of mAbs.
In the later screens samples were subject to prolonged thermal stress at 25oC as a stringent stability test.
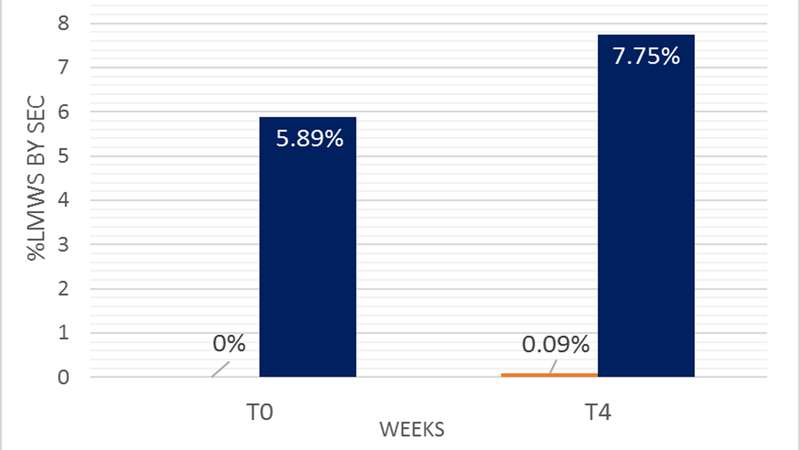
Application of Arecor’s smart formulation technology reduces fragmentation to ≤0.09 per cent even after a four- week hold at 25ºC (Figure shown below).
In addition to temperature stability, the Arecor smart formulation technology also confers improved physical stability
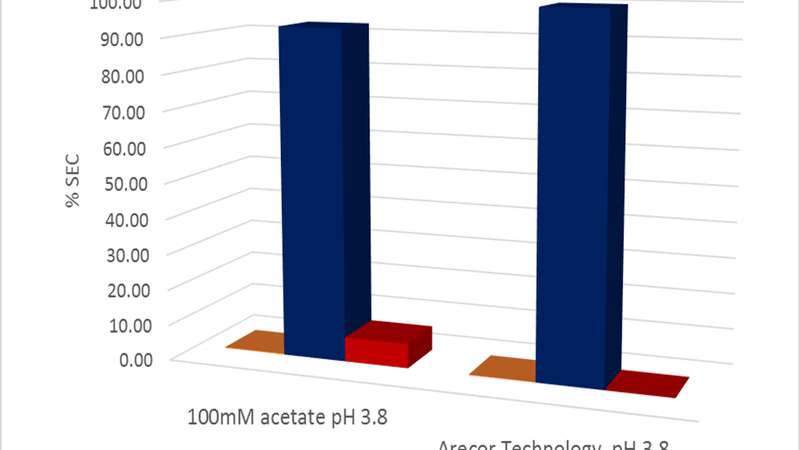
Post application of elution buffer, the protein product was agitated at 100 rpm for three days at 25°C. Under these conditions the Arestat™ protein A elution composition protects against fragmentation and has an eight per cent higher main peak purity potentially improving final downstream yield (Figures shown below).
By using a scale-down volume approach to formulation testing and applying liquid handling robotics a large formulation screen was completed significantly more quickly and with less mAb material than would have been the case with standard approaches.
3. Transfer and scale-up of FDBK, USP, DSP and analytical mAb platform process at CPI
A technology transfer was carried out to transfer the upstream, downstream and analytical methods for mAb production from FDBK to CPI.
The successful transfer of the process was then demonstrated at CPI at a comparable scale of operation as at FBDK. CPI then scaled up to a 200-litre bioreactor scale in order to produce larger amounts of particular DSP intermediates after the product capture, intermediate purification and polishing stages for use in formulation testing in the project.
4. Scale down unit operation testing of downstream processing formulations
The short-listed formulations were tested using high throughput scale down approaches.
For the chromatography steps, robotic liquid handlers (Perkin Elmer Janus systems) installed at CPI were used with Atoll supplied RobocolumnsTM. to evaluate the impact of the new formulations on each chromatography stage.
Formulation chromatographic performance testing was coupled with the application of multiple high-resolution analytical methods to determine the impact of the new formulations on product quality (including elution yield, impurity clearance comparison with controls) as well as the stabilisation of the mAb.
This high throughput approach allowed more new formulations to be tested in a very short time.
Eight experimental conditions could be run at one time with automated rapid data collection using integral plate readers generating elution chromatograms for the experimental formulations.
The columns used contained 0.6ml of chromatography resin and enabled careful control of residence times to mimic the performance of large scale columns.
This scale down approach allowed eight separate conditions to be tested in two hours using <200mg of material.
In contrast, conventional approaches using bench scale chromatography systems would have taken around four days and required around 25-fold more material to collect the same data.
Buffer requirements were also reduced proportionally.
5. Downstream processing formulation improvements
Preliminary data from the project has shown significant improvements in product quality could be obtained through the use of the Arecor formulations compared to industrial standard compositions when mAbs were exposed to stresses such as pH, concentration, and time encountered during typical downstream stages.
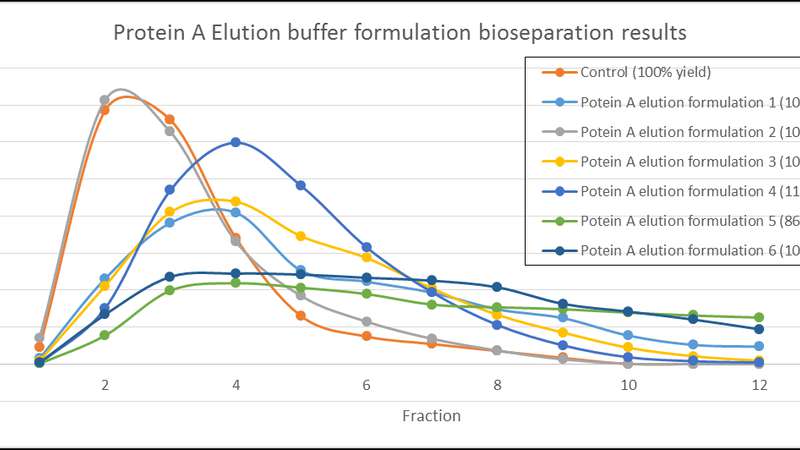
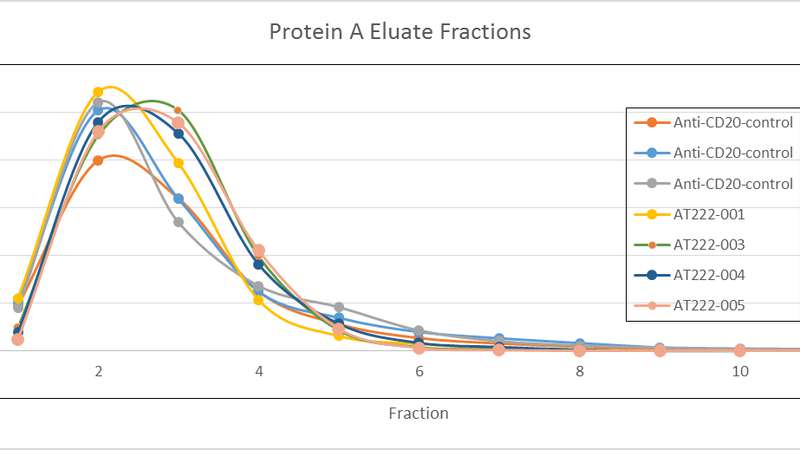
As an example, the potential manufacturing efficiency and quality improvements offered by implementation of Arecor’s smart formulation technology is exemplified by the elution chromatograms generated with different protein A elution buffers.
Initial chromatographic separation profiles, shown in Figure A below, were further improved by small calculated adjustments to the Arecor formulations to ensure a more concentrated elution profile, as seen in Figure B.
Data from the formulations developed initially shows comparable levels of product recovery to control elution formulations with subsequent analysis of elution pools allowing the formulations to be assessed through their impact on important product quality attributes.
Liquid handler as used in the scale down dsp studies
This preliminary data has shown improvement of critical quality attributes (CQA) using the formulations.
Additionally, the formulations do not impact the unit operation performance or in the case of protein A media do not result in increased ligand loss.
Data from these preliminary rounds of unit operation tests is being used in refining the formulations for pilot scale testing to give a set of downstream processing formulations that provide the best product yield and product protection from bioprocessing stresses.
The outcome
With a unique combination of expertise across the consortium, on completion of the project, the collaboration will deliver a novel formulation platform for use in routine biopharmaceutical manufacturing.
The platform will be applied to reduce the production costs of biopharmaceuticals, which will ultimately reduce the cost of therapeutics to healthcare providers.
These new formulations may also enable the production of biotherapeutics that are currently very difficult or impossible to manufacture due to the protein destabilising conditions necessary for their purification. The development of this innovative platform will align with the strategic objective of the IBC scheme, to enhance the UK’s position as a global leader in this sector.
References
- Zola H, Thomas D, Lopez A (Sep 2013). Monoclonal Antibodies: Therapeutic Uses. In: eLS. John Wiley & Sons Ltd, Chichester. https://www.els.net/WileyCDA/ElsArticle/refId-a0002176.html [doi: 10.1002/9780470015902.a0002176.pub3]
Let’s innovate together
To find out more about how we can work together, please enter your details below.