
Biostreamline event showcases project results
28 Jun 2019
An innovative collaboration to streamline the development of cost-effective next generation biotherapeutics hosted a dissemination event at CPI Darlington on the 26th June to showcase the project results.
BioStreamline is a highly innovative, collaborative project involving six partners across the UK including Lonza Biologics, UCB Celltech, Sphere Fluidics, Horizon Discovery, Alcyomics Ltd and CPI. This £11.2m project, applies cutting-edge technologies to overcome some of the most significant shortcomings of the biologics supply chain and deliver cost-effective therapies to patients.
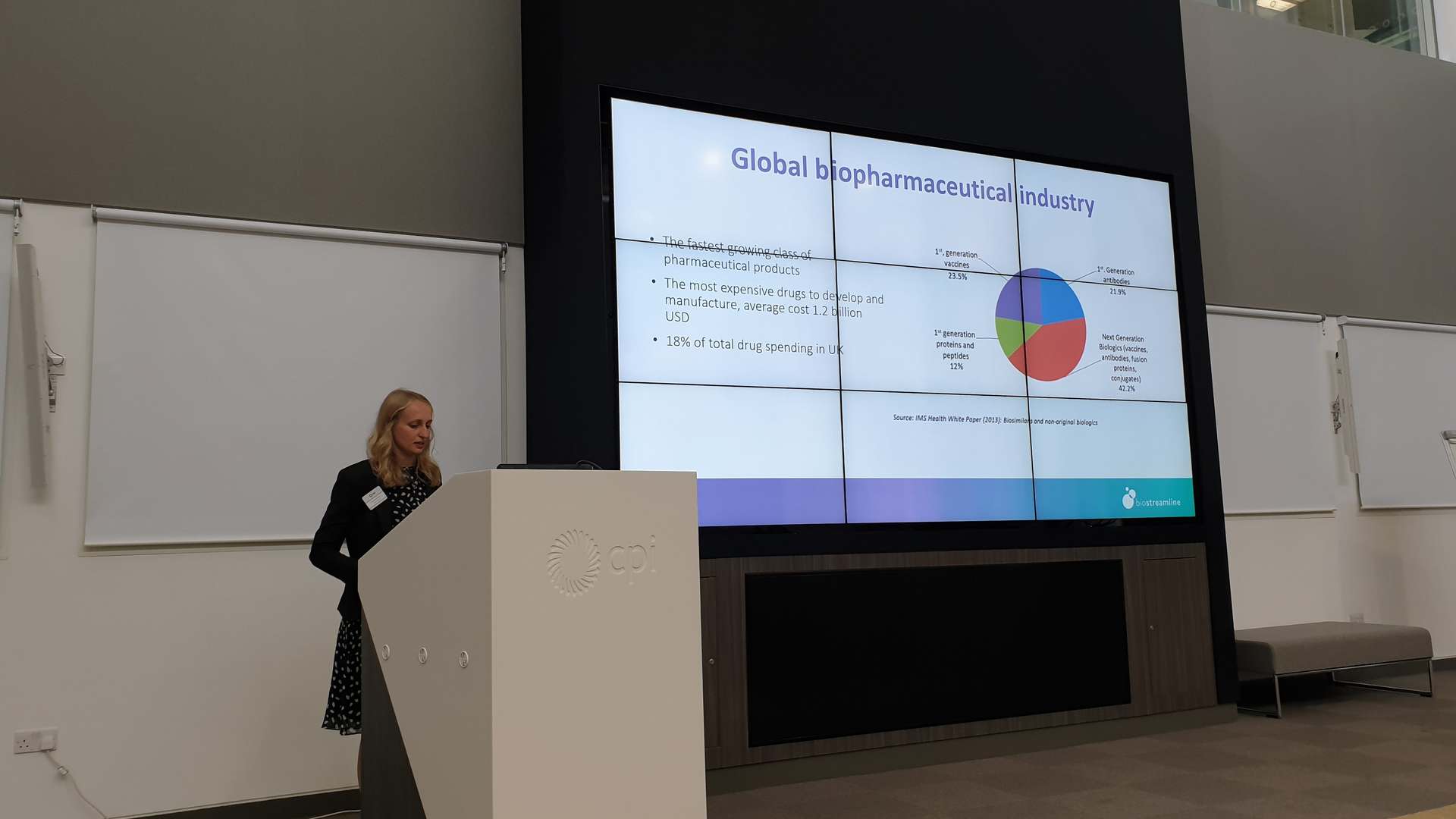
BioStreamline, with a focus on monoclonal antibody therapeutics, aims to overcome such challenges by applying new technologies that will make it easier to identify failing drugs earlier in development, as well as improving development and manufacturing processes.
The project involved the following major areas:
- Development of a new single cell analysis system (Cyto-Mine®) and associated methodologies to enable screening of B‑cells for antibody discovery and analysis of antibody-producing clones for selection of high-producing cell lines. Delivery of this system will increase the speed and efficiency of the entry into the biologics supply chain.
- New approaches to cell line development using genome-wide gene knock-out screening using CRISPR-Cas9 to improve specific cell line features including increased productivity.
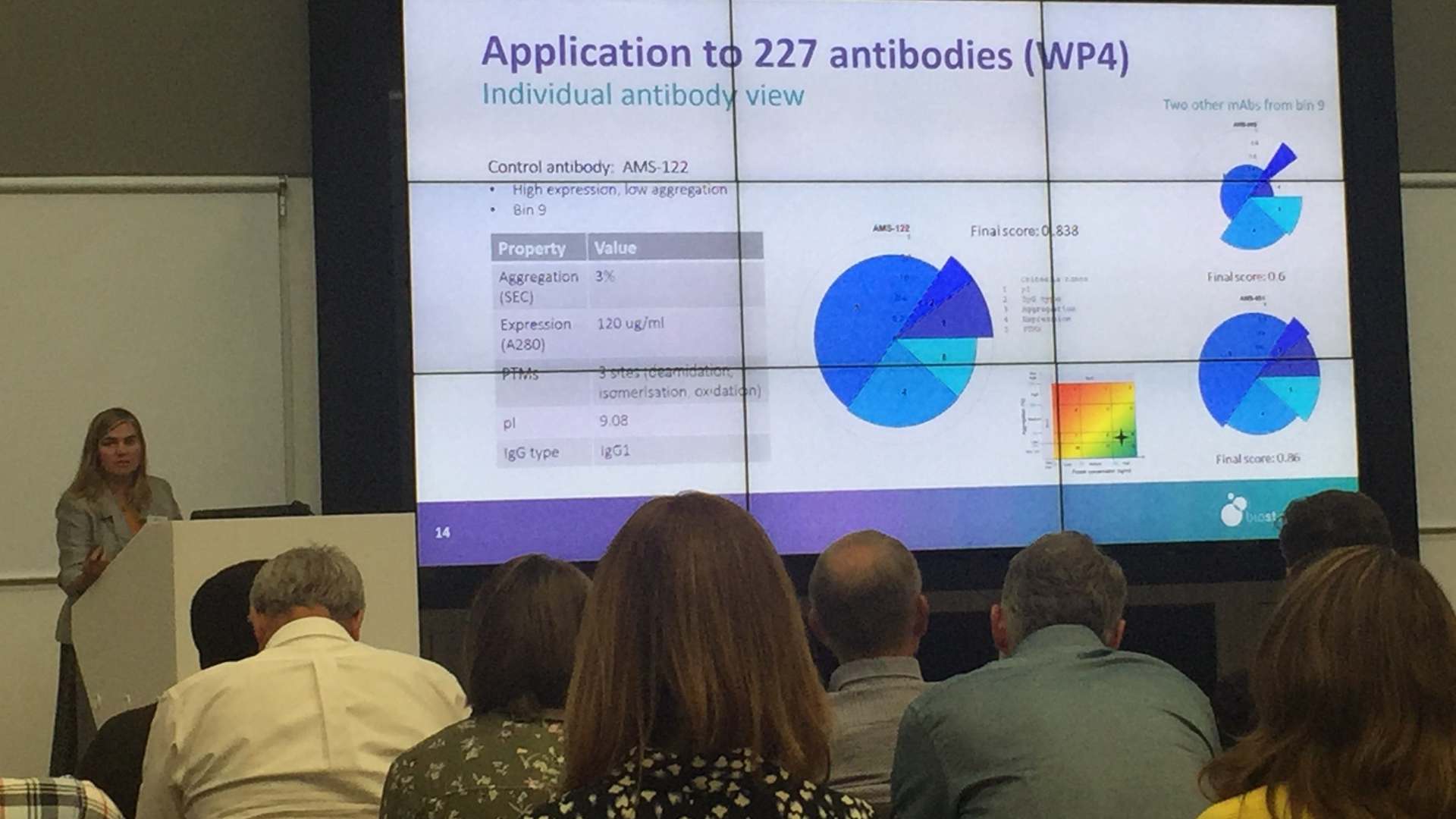
- Creation of an agile, flexible and improved approach to biotherapeutics developability. Following expression and purification of 50+ mAbs using industry leading platforms, data on the biochemical, biophysical and stability of each molecule was collected and together with other inputs lead to the design of a developability tool which will enable more accurate decision making.
- Investigation of clinically informative, easy to implement, preclinical assessment of immunogenicity risks of biotherapeutics to minimise exposure of human patients to potential safety risks during early clinical trial development.
By gaining a better understanding of candidate molecules earlier in the process, manufacturers will be able to make more informed decisions, reducing drug development risk and provide more efficient use of time and resources.
The collaboration partners presented a series of talks showcasing the results achieved through the course of this major project.






Let’s innovate together
To find out more about how we can work together, please enter your details below.